As discussed earlier, seawater is nearly incompressible. However, when it comes to a discussion of stability of the stratification at great depth, the effect of compressibility has to be taken into account.
The temperature of a gas or fluid increases under pressure. For pure water, the rate of temperature increase as a result of a pressure increase, called the adiabatic lapse rate, is 0.035°C per 1000 m increase in depth. This rate is only marginally larger for seawater. Given the total ocean depth of less than 12 km, the maximum adiabatic warming that can be achieved by increasing pressure is less than 1°C.
|
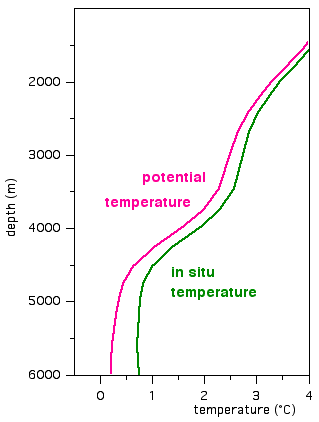
Over most of the ocean's depth, the adiabatic temperature increase with pressure (ie depth) is much smaller than the temperature decrease produced by other factors (eg solar heating at the surface) and can safely be neglected. This can be seen in the diagram on the left, which shows an enlargement of the temperature profile from the Atlantic Ocean at 10°S:
Above 4500 m depth, the observed temperature, also called the in situ temperature T (in situ: Latin for "on location") decreases with depth at a rate of about 1°C per 1000 m (from 3°C at 2500 m depth to 1°C at 4500 m). This rate of change is much larger than the adiabatic lapse rate.
We obtain the potential temperature, usually given the symbol Θ (Theta), by subtracting the temperature increase produced by the compression of the water. The potential temperature is thus the temperature which the water would have when brought up to the surface (ie to zero pressure).
Above 4500 m depth, the curves of potential temperature and in situ temperature look very similar, the only difference being that Θ is slightly lower than T.
Below 4500 m the temperature of the ocean becomes extremely uniform. Temperature changes are so small that the in situ temperature T eventually increases with depth, reflecting only the adiabatic temperature increase with pressure. This could be seen as a sign for an unstable stratification, since density decreases as temperature increases.
However, the stratification is still stable, since any water moved from, say, 5000 m to 6000 m depth would not be colder than its environment because it would increase its temperature in accordance with the increase in pressure.
We can obtain a correct appreciation of stability if we eliminate the effect of pressure on temperature by using potential temperature Θ. The diagram on the left demonstrates that Θ decreases with depth over the entire water column, while T shows an increase with depth below 5500 m. The decrease of Θ with depth indicates that the water is stably stratified.